Liposomal delivery of bacteriophages to target intracellular bacterial pathogens
State of the art
Humankind is heading towards an imminent antibiotics crisis, due to the rapid increase of developing resistances in bacteria towards antibiotics [1]. For that reason, we urgently need to find alternative ways to continue the successful treatment of bacterial infections. The bearer of hope in fighting even heavily antibiotic-resistant bacteria are bacteriophages (hereinafter referred to as phages) – viruses, which specifically infect certain bacteria [2-4]. In the last few years, phages have been repeatedly proposed as a well-tolerated alternative to antibiotics to confront this emerging threat of antibiotic-resistant bacterial infections [2-5]. Historically, phages were used for therapeutical purposes in many countries, including the Western world. Most experience, however, has been gained by the former Soviet Union and Eastern European countries for about 100 years by now [2,4]. For example, the 1923 founded Eliava Institute in Tbilisi, Georgia, was the first clinical center specialized on phage-based treatment of bacterial infections, practicing up to the present day [6-7]. However, a particular challenge is the treatment of intracellular bacteria, because those pathogens are able to hide inside their hosts, and by that escape from the immune system and most drugs to a great extent [8-9]. Since bacteriophages cannot diffuse through the plasma membrane of cells, strategies are needed to overcome this major obstacle. Broxmeyer and colleagues demonstrated with their Trojan Horse strategy the successful treatment of Mycobacterium tuberculosis within host cells. For this, they used non-pathogenic Mycobacterium smegmatis to transport the lytic mycobacteriophage TM4 into macrophages infected with M. tuberculosis [10].
In our studies, and within the framework of the RTG 2202 ‘Transport across and into membranes’, we propose a lipid-based delivery of phages, without the use of a bacterial host as carrier. Those liposomal systems are well investigated, and seem to be low in severe side-effects [11].
Research objectives
Previous studies of our laboratory have successfully shown the encapsulation of phages within liposomes [12]. However, the encapsulation efficacy of phages has still to be improved. For the realization of our intracellular phage-therapy approach, we will pursue two strategies to overcome the cell’s plasma membrane: membrane fusion and endocytosis (see Figure 1).
This project can be divided into the following steps:
(1) By using cationic and cone-shaped lipids, both facilitating membrane fusion, we can generate liposomes for cargo delivery by direct fusion with the plasma membrane (preliminary data, [13]).
(2) Lipids that trigger phagocytosis/endocytosis are used to induce uptake by macrophages (preliminary data).
(3) In addition, we will apply our knowledge about lectins (i.e. carbohydrate-binding proteins) acquired during the first RTG funding period. Several natural lectins have been used to crosslink two populations of giant liposomes in order to mimic cell-cell interactions and cell adhesion [14]. As next step, tailor-made bifunctional lectins have been developed [15], which induce membrane fusion (preliminary data). Phage-containing liposomes will be decorated with fusogenic lectins, which by binding to receptors at the host cell plasma membrane may induce the fusion of the liposome with the host cell plasma membrane, draining the phages into the host cell cytosol.
(4) The lower pH within the early endosome will allow us to release phages either into the endosome lumen by liposome disintegration or into the cytosol by endosome-liposome fusion (Figure 1). Both attempts can be achieved by introducing certain pH-sensitive lipids into the lipid composition.
Using the above-mentioned uptake strategies, we will be able to deliver phages into intracellular compartments, such as bacterial phagosomes, and into the cytoplasm - both locations of intracellularly hiding bacteria.
(5) The final step will be the exact localization of delivered phages as well as their co-localization with pathogenic bacteria. We will choose two bacteria, Listeria monocytogenes and Mycobacterium smegmatis for our proof of concept studies, and confirm potential contacting and elimination of the bacteria by microbiological, cell biological, biochemical and imaging approaches.
In summary, the main goal of this study is to reduce and eventually eliminate bacterial pathogens (Listeria and Mycobacteria) inside infected, living cells using liposomal delivery of encapsulated bacteriophages by direct membrane fusion or endocytosis.
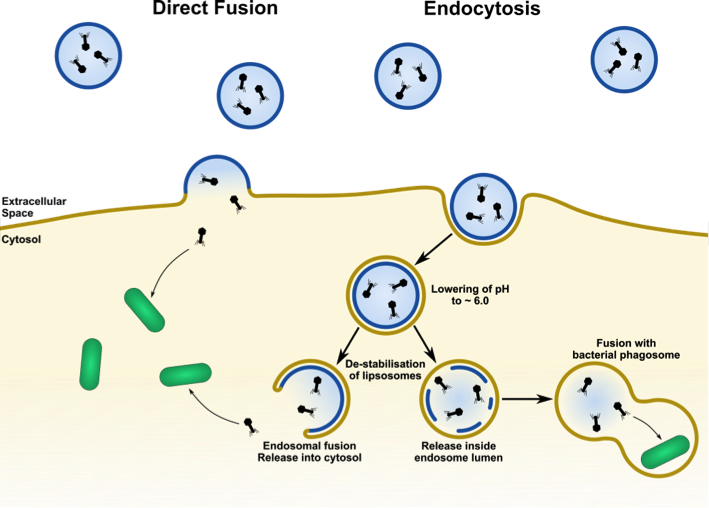
Figure 1: Principles of liposomal delivery of bacteriophages to target intracellular bacteria.
References
[1] Rodríguez-Rojas et al. (2013) Int. J. Med. Microbiol. 303(6-7): 293-7
[2] Hanlon (2007) Int. J. Antimicrob. Agents 30(2): 118-28
[3] Yosef et al. (2014) Bacteriophage 4(1): e28491
[4] Sulakvelidze et al. (2001) Antimicrob Agents Chemother. 45(3): 649-59
[5] Romero-Calle et al. (2019) Antibiotics (Basel) 8(3). pii: E138
[6] Kutateladze (2015) Virol Sin. 30(1): 80-1
[7] Parfitt (2005) Lancet 365(9478): 2166-7
[8] Ray et al. (2009) Nat Rev Microbiol. 7(5): 333-40
[9] Case & Samuel (2016) Microbiol Spectr. 4(1): 667–92
[10] Broxmeyer et al. (2002) J Infect Dis. 186(8): 1155-60
[11] Torchilin (2006) Annu. Rev. Biomed. Eng. 8: 343-75
[12] Nieth at al. (2015) Expert Opin. Drug Deliv. 12(9): 1411-24
[13] Csiszár et al. (2010) Bioconjug Chem. 21(3): 537-43
[14] Villringer et al. (2018) Sci Rep. 8(1): 1932
[15] Ribeiro et al. (2018) Chem Sci. 9: 7634-41
Publications
[1] Zheng S, Eierhoff T, Aigal S, Brandel A, Thuenauer R, de Bentzmann S, Imberty A, Römer W. 2017. The Pseudomonas aeruginosa lectin LecA triggers host cell signalling by glycosphingolipid-dependent phosphorylation of the adaptor protein CrkII. Biochim Biophys Acta Mol Cell Res. 1864(7): 1236-1245
[2] Villringer S, Madl J*, Sych T, Manner C, Imberty A, Römer W*. 2018. Lectin-mediated protocell crosslinking to mimic cell-cell junctions and adhesion. Sci Rep. 8(1): 1932
[3] Ribeiro JP, Villringer S, Goyard D, Coche-Guerente L, Höferlin M, Renaudet O, Römer W*, Imberty A*. 2018. Tailor-made Janus lectin with dual avidity assembles glycoconjugate multilayers and crosslinks protocells. Chem Sci. 9: 7634-41 * corresponding author
[4] Wilhelm I, Levit-Zerdoun E, Jakob J, Villringer S, Frensch M, Übeopolhart R, Landi A, Müller P, Imberty A, Thuenauer R, Claudinon J, Jumaa H, Reth M, Eibel H, Hobeika E*, Römer W*. 2019. Carbohydrate-dependent B cell activation by fucose-binding bacterial lectins. Sci Signal. 12(571): eaao7194
[5] Thuenauer R*, Landi A, Trefzer A, Altmann S, Wehrum S, Eierhoff T, Diedrich B, Dengjel J, Nyström A, Imberty A, Römer W*. 2020. The Pseudomonas aeruginosa lectin LecB causes integrin internalization and inhibits epithelial wound healing. mBio. 11(2): e03260-19.
[6] Darkow E, Rog-Zielinska EA, Madl J, Brandel A, Siukstaite L, Omidvar R, Kohl P, Ravens U, Römer W and Peyronnet R. 2020. The lectin LecA sensitizes the human stretch-activated channel TREK-1 but not Piezo1 and binds selectively to cardiac non-myocytes. Front. Physiol. 11: 457.
[7] Schubert T, Sych T, Madl J, Xu M, Omidvar R, Patalag LJ, Ries A, Kettelhoite K, Brandel A, Mely Y, Steinem C, Werz DB, Thuenauer R, Römer W. 2020. Differential recognition of lipid domains by two Gb3-binding lectins. Sci Rep. 10: 9752
[8] Brandel A, Aigal S, Lagies S, Schlimpert M, Lehmann A, Hummel D, Fisch D, Meléndez AV, Madl J, Eierhoff T, Kammerer B, Römer W. 2020. The Gb3-enriched CD59/flotillin plasma membrane domain regulates host cell invasion by Pseudomonas aeruginosa. bioRxiv, doi: https://doi.org/10.1101/2020.06.26.173336
Contact
Prof. Dr. Winfried Römer
Institute of Biology II and Centre for Biological Signalling Studies (BIOSS)
Biologie II, Signalhaus
Schänzlestr. 18
79104 Freiburg
Phone: +49 (761) 203 67500
Fax: +49 (761) 203 67535
winfried.roemer@bioss.uni-freiburg.de
https://www.bioss.uni-freiburg.de/prof-winfried-roemer/



