Unusual Ammonium Transporters
Ammonium transport (Amt) proteins are ubiquitous integral membrane proteins that transport NH4+ across cell membranes. Amt proteins involved in the uptake of NH4+ as direct N-source for biosynthesis are present in prokaryotes, archaea and plants [1]. In mammals, Rhesus proteins are the Amt homologs and their presence in erythrocyte, kidney and liver cell membranes can be directly related to NH3/NH4+ detoxification and pH homeostasis processes [2]. Long recognized for their immunogenic characteristics and involvement in hemolytic diseases of infants, a number of genetic diseases have been associated with the inability to correctly express Rh proteins [2,3]. Understanding the molecular details of transport and regulation of Amt proteins is therefore highly relevant to help treat and solve many human Rh-related diseases.
A significant amount of our research has focused on the archaea homologs, in particular on the Af-Amt1 protein from Archaeoglobus fulgidus. We have solved the crystal structures of native [4] and of several protein variants up to 1.3 Å resolution and recently proved that these proteins function as slow electrogenic transporters, with a turnover rate of 10E1 – 10E2 ions/sec/trimer [5]. With three-dimensional high-quality structures and a reliable in vitro functional assay at hand, interesting mechanistic questions can now be addressed to e.g. validate the role of precise amino acid residues or the existence of conformational changes during transport events (Figure 1).
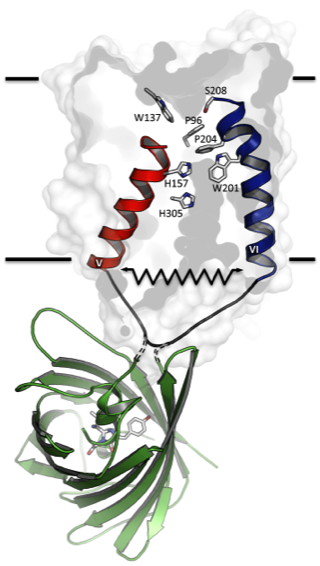
Figure 1: Assessment of conformational changes in Amt proteins using a fluorescent reporter. Scheme of Arabidopsis thaliana Amt1;3 monomer (white surface model) fused to a Green Fluorescent Protein (green cartoon model) at the cytoplasmic loop-5. Loop-5 connects transmembrane helices V (red) to VI (blue), both of which hold highly conserved and functionally relevant residues (stick representation according to Af-Amt1 nomenclature [4]). Using such a construct we were able to monitor specific fluorescence changes that were directly correlated to the Amt domain transport activity in living cells. Possible conformational changes are depicted by a spring [6].
The main goal of this project is therefore to elucidate how ammonium transport proteins work at atomic detail. The student engaged in this investigation will use state-of-the-art biochemical and biophysical technologies to derive structural and functional information on various protein constructs.
References
[1] Andrade SLA, Einsle O (2007) Molec Memb Biol 24:357-365.
[2] Biver S, et al (2008) Nature 456:339-343.
[3] Huang CH (1998) J Mol Biol 273:2207-2213.
[4] Andrade SLA, Dickmanns A, Ficner R, Einsle O (2005) Proc Natl acad Sci USA 102:14994-14999.
[5] Wacker T, Garcia-Celma JJ, Lewe P, Andrade SLA (2014) PNAS 111:9995-10000.
[6] De Michele R, et al (2013) eLife 2:e00800.
Contact
Prof. Dr. Susana Andrade
Institute of Biochemistry
Albertstr. 21
79104 Freiburg
Phone: +49 (761) 203 8719
Fax: +49 (761) 203 6161
andrade@bio.chemie.uni-freiburg.de
http://www.biophysics.uni-freiburg.de



