Lipid bilayer effects on membrane transport in biophysics and pharmaceutics
Most drugs and many biomolecules cross cellular membranes by partitioning into and diffusion through the lipid matrix. Kinetics and thermodynamics of hydrophobic or amphiphilic ligands or substrates binding to membrane proteins depend on their interaction with the lipid bilayer. Biologically active molecules may act by changing the properties of the lipid matrix, including permeability, lipid asymmetry, order, dynamics, and lateral organization that modulate membrane protein function or membrane barrier integrity [1]. We are interested in a better fundamental understanding of the mechanistic and thermodynamic processes governing these phenomena.
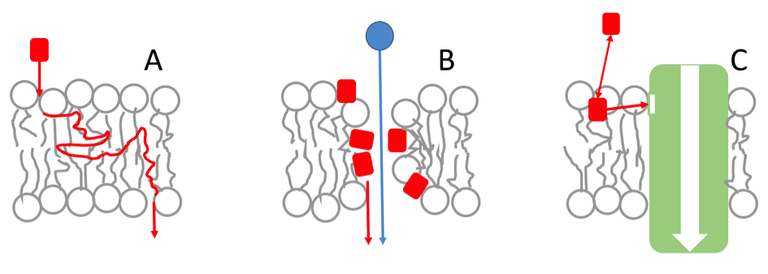
Fig.1: Schematic representation of lipid matrix effects on membrane transport. (A) Most drugs can cross membranes by diffusion across the lipid bilayer. (B) Membrane-active compounds may act by altering membrane permeability and other membrane properties. (C) The function of membrane proteins may be affected by lipid matrix effects governing the local concentration and dynamics of lipophilic and amphiphilic ligands and their accessibility to membrane-buried binding sites. Furthermore, the functional state of proteins and protein assemblies may respond to the composition and overall properties of the lipid matrix.
The detailed, quantitative and mechanistic understanding of membrane-active compounds has been a long-term aim of the lab. These compounds, including antimicrobial and cell penetrating peptides, bacterial lipopeptides, synthetic antiseptics, preservatives, pharmaceutical penetration enhancers, and others enter the membrane from solution and alter its permeability and other properties. They play important roles in biological processes and have a multitude of applications in medicine, pharmaceutics, biotechnology, crop protection, and other fields. Current work is mainly dedicated to the interplay between membrane affinity and local membrane perturbation of bacterial, cyclic lipopeptides, which need to be understood for a proper design and interpretation of studies on model systems to address biological questions.
Particular emphasis is paid to an improvement of model membranes for a deeper understanding of membrane processes on a quantitative, biophysical level and their critical assessment with respect to their relevance and limitations to demonstrate biomembrane function. A particular focus has been on lipid-asymmetric (proteo)liposomes [2].
This approach is supported by efforts to implement new experimental assays to study membrane permeation, perturbation, and permeabilization based on cutting edge microcalorimetry (ITC, DSC, PPC), subnanosecond time-resolved fluorescence spectroscopy, [3] and other techniques.
Finally, our strategy is to utilize principles of transmembrane transport of drugs to allow for the optimal loading, retainment, and triggered release of drugs out of liposomal drug delivery systems [4].
References
[1] The mechanism of membrane permeabilization by peptides: Still an enigma. W.C. Wimley and K. Hristova (2020) Australian J. Chem. 73:96-103
[2] Stairway to Asymmetry: Five Steps to Lipid-Asymmetric Proteoliposomes. Markones M, Fippel A, Kaiser M, Drechsler C, Hunte C, Heerklotz H. (2020) Biophys J. 3495:34343-7.
[3] Biomembrane Permeabilization: Statistics of Individual Leakage Events Harmonize the Interpretation of Vesicle Leakage. S. Braun S, Š. Pokorná, R. Šachl, M. Hof, H. Heerklotz, M. Hoernke (2018) ACS Nano 12:813-819
[4] Thermosensitive liposomal drug delivery systems: state of the art review. B. Kneidl, M. Peller, G. Winter, L. Lindner, and M. Hossann (2014) Intl. J. Nanomedicine 9:4387-4398)
Contact
Prof. Dr. Heiko Heerklotz
Institute for Pharmaceutical Sciences - Pharmaceutics division
Hermann-Herder-Str. 9
79104 Freiburg
Phone: +49 (761) 203 6336
Fax: +49 (761) 203 6357
heiko.heerklotz@pharmazie.uni-freiburg.de
http://www.pharmtech.uni-freiburg.de



