Structure and function of secondary transport proteins
Secondary active transport proteins fulfil an essential role in the specific translocation of hydrophilic and/or charged compounds across biological membranes. The mechanistic coupling to an energetically downhill transport of ions allows it to accumulate its substrate against a concentration gradient, and this principle is employed by a multitude of active uptake systems in all kingdoms of life.
Among other systems we are studying the formate/nitrite transporter (FNT) family of integral membrane proteins in collaboration with Susana Andrade, in order to understand both their molecular architecture and their physiological functionality in atomic detail. The FNT family comprises both ion channels that mediate the rapid, but passive conduction of monovalent anions along their concentration gradient, and intricate, bifunctional channel/transporters that are able to switch their mode of operation dependent on external stimuli such as the pH value. In enterobacteria, the nitrite channel NirC is an assimilatory uptake channel for nitrite, a possible source of nitrogen for biosynthesis. However, it serves a dual function in that it also imports the reactive peroxynitrite for cytoplasmic detoxification, a leukocyte cytotoxin produced as part of the innate immune response. For Salmonella, the presence of NirC directly relates to the pathogenicity of the bacterium, making it a relevant target for the development of new antibiotics. The structure of NirC showed the typical, pentameric symmetry of FNT channels that contain a single substrate channel in each protomer [1]. Within the channel, two hydrophilic constrictions separate a central vestibule that an anion will only be able to overcome after protonation to a neutral state [2].
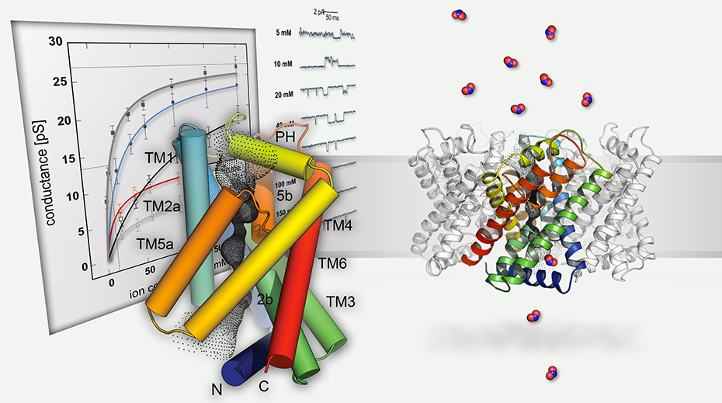 Figure 1: Structural and functional analysis of FNT family channels and transporters. The proteins form protomers with six transmembrane helices that surround a central channel (left). The pentameric nitrite channel NirC, a member of the FNT family, mediates the import of nitrite anions into the cytoplasm for reduction to bioavailable ammonium (right).
Figure 1: Structural and functional analysis of FNT family channels and transporters. The proteins form protomers with six transmembrane helices that surround a central channel (left). The pentameric nitrite channel NirC, a member of the FNT family, mediates the import of nitrite anions into the cytoplasm for reduction to bioavailable ammonium (right).
While FNT proteins fulfil essential physiological tasks in mediating anion permeation, one of its members, the formate channel FocA, takes on an intriguing dual role. In the human intestinal tract, enteric bacteria thrive in a nutrient-rich environment. They predominantly use glucose as a carbon and energy source, but lack sufficient O2 for the complete oxidation to CO2. Therefore, their preferred metabolism is mixed-acid fermentation, in which the glycolysis end product pyruvate is further broken down into formate, acetate and lactate. Initially, the bulk product formate, HCOO–, is exported to the environment via the formate channel FocA, but over time this leads to a substantial acidification of the medium. In active cultures the pH can quickly drop below 4, becoming prohibitive for further organismic growth. The bacteria then undergo a remarkable metabolic switch, where they re-import formic acid (now protonated at low pH) for an internal disproportionation to yield the gases H2 and CO2. Interestingly, this switch is mediated by the same FocA protein, whose unique ability is to reverse their functionality in a pH-dependent manner, changing from passive exporters for the formate anion to secondary active importers for formic acid. Our structural and electrophysiological analysis has revealed that this switch occurs at a sharply defined pH value and is connected to a conformational change in the N-terminal helix of each transporter protomer [3]. While the dual functionality seems to be unique to FocA, most FNT proteins – and in fact many anion channels – exhibit a broad substrate specificity. Their cargo is always monovalent anions, but spans a range of sizes and chemical properties. For the proteins, that importantly and efficiently exclude water from crossing the membrane, this requires a substantial flexibility in their selectivity filter. In FNT proteins, this is likely provided by the connected arrangement of two hydrophobic constrictions that can successively expand for a cargo molecule, without granting passage to water. When screening the substrate spectrum of FocA by direct electrophysiology, we found that FocA not only exports formate, but also the other acids produced by the bacteria, lactate and acetate, and even does so at rates that correspond to the amounts at which they are produced in mixed-acid fermentation [4]. This underlines the impressive flexibility and specificity of membrane transport proteins that we only begin to unravel in our studies.
References
[1] Lü, W., Du, J., Schwarzer, N. J., Wacker, T., Andrade, S. L. A., and Einsle, O. (2013) Biol. Chem. 394, 715-727
[2] Lü, W., Schwarzer, N. J., Du, J., Gerbig-Smentek, E., Andrade, S. L. A., and Einsle, O. (2012) Proc. Natl. Acad. Sci. U. S. A. 109, 18395-18400
[3] Lü, W., Du, J., Wacker, T., Gerbig-Smentek, E., Andrade, S. L. A., and Einsle, O. (2011) Science 332, 352-354
[4] Lü, W., Du, J., Schwarzer, N. J., Gerbig-Smentek, E., Einsle, O., and Andrade, S. L. (2012) Proc. Natl. Acad. Sci. U. S. A. 109, 13254-13259
Publications
Ilcu, L, Röther, W, Birke, J, Brausemann, A, Einsle, O & Jendrossek, D (2017) Structural and functional analysis of latex clearing protein (Lcp) provides insight into the enzymatic cleavage of rubber. Sci. Rep., 7, 6179.
Contact
Prof. Dr. Oliver Einsle
Institute of Biochemistry
Albertstr. 21
79104 Freiburg
Phone: +49 (761) 203 6058
Fax: +49 (761) 203 6161
einsle@biochemie.uni-freiburg.de
http://www.xray.uni-freiburg.de



