Novel factors involved in transport of proteins to respiratory chain complexes
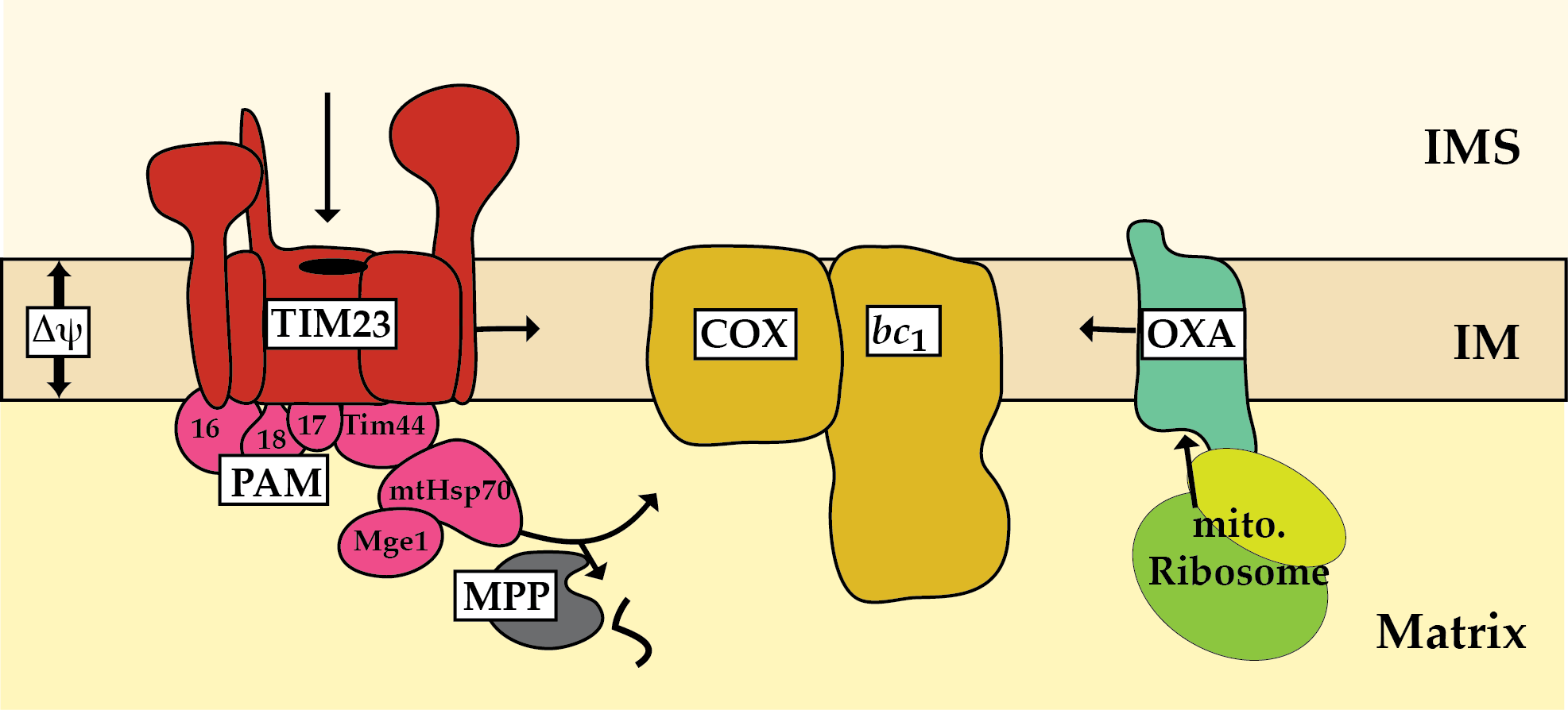
The mitochondrial cytochrome c oxidase (complex IV; COX) of the respiratory chain transfers electrons from cytochrome c to oxygen. The activity of the respiratory chain complexes generates a proton-gradient across the inner membrane, which is used by the ATP-synthase to produce ATP for cellular metabolism. In baker´s yeast Saccharomyces cerevisiae complex IV forms a supercomplex with cytochrome c reductase (complex III, bc1) and consists of eight nuclear-encoded and three mitochondrially-encoded subunits. The formation of complex IV is crucial for respiratory growth. The translocase of the outer membrane (TOM complex) imports precursors of nuclear-encoded COX subunits into mitochondria. Subsequently, the presequence translocase (TIM23 complex) transports the majority of these precursor proteins into or across the inner membrane in a membrane potential dependent manner. The presequence translocase-associated motor (PAM) drives the translocation into the mitochondrial matrix. The ATPase activity of the mitochondrial Hsp70 is crucial to complete protein translocation. Mitochondrially-encoded proteins are co-translationally integrated into the inner membrane via the oxidase assembly (OXA) machinery. Subsequently, the single COX subunits associate via distinct assembly intermediates to form the mature complex. Thereby, a plethora of proteinaceous factors mediate the stepwise formation of respiratory chain complexes. How import and assembly of nuclear-encoded complex IV subunits are coordinated to avoid accumulation of unassembled precursors is poorly understood. We found that mtHsp70 and its cofactor Mge1 promote transport of the peripheral subunit Cox4 to assembly intermediates of the cytochrome c oxidase. Furthermore, subunits of the TIM23/PAM complex associates to respiratory chain supercomplexes and assembly intermediates. These observations point to close link between protein import and the formation of the respiratory chain complexes in mitochondria. In this project we will analyse novel factors involved in the assembly of respiratory chain complexes. Particularly, we will focus on the coordination of transport of precursors of COX subunits across and into the inner membrane and their subsequent assembly into the functional complex.
References
[1] Böttinger L, Guiard B, Oeljeklaus S, Kulawiak B, Zufall N, Wiedemann N, Warscheid B, van der Laan M, Becker T (2013) A complex of Cox4 and mitochondrial Hsp70 plays an important role in the assembly of the cytochrome c oxidase. Mol. Biol. Cell. 24:2609-2619.
[2] Qiu J, Wenz LS, Zerbes RM, Oeljeklaus S, Bohnert M, Stroud DA, Wirth C, Ellenrieder L, Thornton N, Kutik S, Wiese S, Schulze-Specking A, Zufall N, Chacinska A, Guiard B, Hunte C, Warscheid B, van der Laan M, Pfanner N, Wiedemann N, Becker T (2013) Coupling of mitochondrial import and export translocases by receptor-mediated supercomplex formation. Cell 154:596-608.
[3] Wenz LS, Ellenrieder L, Qiu J, Bohnert M, Zufall N, van der Laan M, Pfanner N, Wiedemann N, Becker T. (2015) Sam37 is crucial for formation of the mitochondrial TOM-SAM supercomplex, thereby promoting b-barrel biogenesis. J. Cell Biol. 210:1047-1054.
Publications
- Krüger, V*, Becker, T*, Becker, L, Montilla-Martinez, M, Ellenrieder, L, Vögtle, FN, Meyer, H, Ryan, M, Wiedemann, N, Warscheid, B, Pfanner, N, Wagner, R and Meisinger, C (2017) Identification of new channels by systematic analysis of the mitochondrial outer membrane. J. Cell Biol. doi.org/10.1083/jcb.201706043 (*equal contribution)
- Ellenrieder, L, Rampelt, H and Becker, T (2017) Connection of protein transport and organelle contact sites in mitochondria. J. Mol. Biol. 429, 2148-2160.
Contact
PD Dr. Thomas Becker
Institute for Biochemistry and Molecular Biology
Stefan-Meier-Str. 17
79104 Freiburg
Phone: +49 (761) 203 5243
Fax: +49 (761) 203 5261
thomas.becker@biochemie.uni-freiburg.de
http://www.biochemie.uni-freiburg.de/ag/becker



