Stoichiometry of heteromeric assemblies across TRP channel families
Transient receptor potential (TRP) channels are a family of structurally similar ion channels that mediate different sensations like touch and temperature, and are responding to various ligands. They assemble as tetramers and are permeable to mono- and divalent cations with ion selectivities depending on the expressed subunit combination. The classification of TRP channel subunits into the groups TRPC, TRPV, TRPM, TRPA, TRPP, and TRPML is primarily based on sequence homology, but also functional similarities can be observed within each group.
Co-assembly of members of different groups into one channel has been observed in several instances (Fig. 1). In contrast to many other ion channels, TRP channels seem quite promiscuous in the choice of their assembly partners. The formation of heteromeric channels implies a mixing of the properties of the different subunits into one protein, i.e. the activation modalities (e.g. ligand binding, temperature range) and conduction properties of the involved subunits will be combined, an effect of high biological relevance.
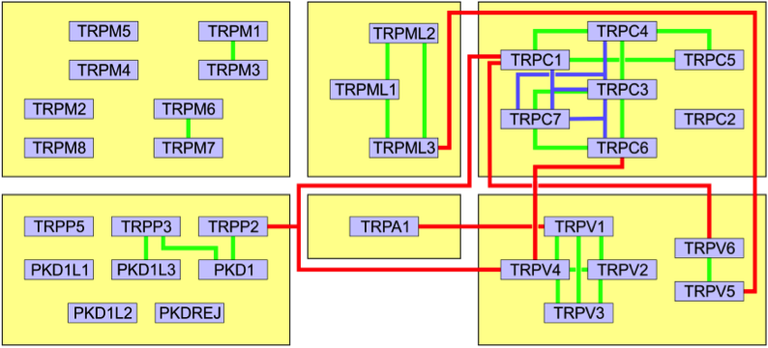
Figure 1: Interactions between the TRP channel members and groups. Green lines: Di-heteromeric interactions within one group. Blue lines: Tri-heteromeric interactions within one group. Red lines: Interactions between channels from different groups.
Several heteromeric assembly possibilities of TRPs from the same or different groups have been suggested previously, e.g. TRPP2 – V4 – C1 [1]; however, the stoichiometry and a potential preference for certain subunit combinations remains unknown. Despite the multitude of known possible combinations, a unifying pattern or deeper understanding of the underlying determinants is missing. The aim of the project is to elucidate the assembly rules for heteromeric TRP channels, decipher the stoichiometry of those assemblies, identify molecular determinants that guide the assembly, and characterize the functional consequences of heteromer formation. We will use a live cell single molecule imaging technique for subunit counting that is based on the identification of photobleaching steps from fluorescent labels fused to membrane proteins of interest (Fig. 2) [2]. This approach allows determining the stoichiometry of membrane protein assemblies containing two or more constituents, and even the detection of a potential preference for certain stoichiometries over others [3].
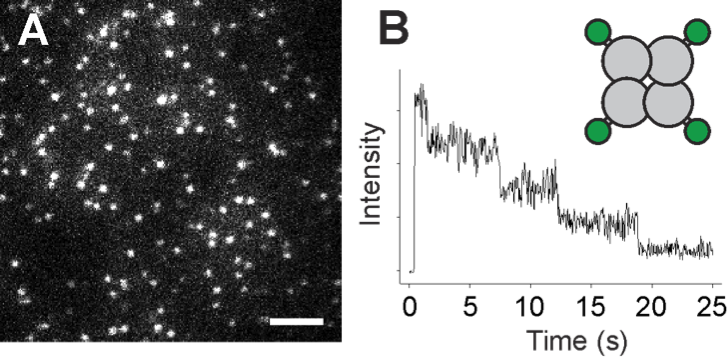
Figure 2: Counting subunits of a protein complex by single molecule imaging of bleaching steps. (A) An image of a Xenopus oocyte showing many individual complexes (bright spots) of a tetrameric membrane protein labeled by GFP. (B) Since every protein complex contains four GFP tags, photobleaching of the GFP results in a stepswise intensity decay, with the number of tags equalling the number of subunits.
|
|
References
[1] Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X (2014) FASEB J. 28:4677-4685.
[2] Ulbrich MH, Isacoff EY (2007) Nat. Methods 4:319-321.
[3] Bartoi T, Augustinowski K, Polleichtner G, Gründer S, Ulbrich MH (2014) Proc. Natl. Acad. Sci. U. S. A. 111:8281-8286.
Contact
PD Dr. Maximilian Ulbrich
Institute of Physiology II and Centre of Biological Signalling Studies (BIOSS)
Physiologie, Signalhaus
Schänzlestr. 18
79104 Freiburg
Phone: +49 (761) 203 97183 (phone)
Fax: +49 (761) 203 97232 (fax)
max.ulbrich@bioss.uni-freiburg.de
http://www.ulbrich-lab.com/index.html



