Protein transport across and into the mitochondrial outer membrane
More than 99% of mitochondrial proteins are encoded in the nucleus as preproteins and have to be sorted into the organelle after their synthesis on cytosolic ribosomes. Transport across the outer membrane is mediated by the TOM-complex (translocase of the outer membrane) which represents the central entry gate for virtually all mitochondrial preproteins. A coordinated action of the TOM-complex with further protein translocases is required to sort mitochondrial preproteins to one of the four mitochondrial subcompartments [1]. While the composition and function of mitochondrial protein import systems had been analyzed in great detail basically nothing was known so far about how these machineries can regulate protein import, i.e. to adapt the import rate to metabolic changes in the cell.
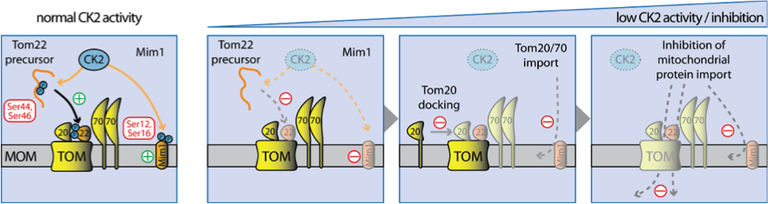
Figure 1: Regulation of the preprotein translocase of the outer membrane (TOM-complex) by casein kinase 2 (CK2)
One important regulatory mechanism therefore could be reversible phosphorylation. However, regulation of mitochondrial functions by posttranlational modifications had been largely neglected in the past due to the assumption that these double membrane and bacterial derived organelles must work quite autonomously. This project aims to study the role of phosphorylation in sorting and assembly of b-barrel proteins of the mitochondrial outer membrane. We have analyzed purified mitochondrial outer membrane fractions of Saccharomyces cerevisiae for posttranslational modifications and identified more than 100 different phosphorylation sites (in collaboration with Prof. A. Sickmann, ISAS, Dortmund). More than 30 of these phosphorylation sites were found at the TOM-complex and located mostly at the cytosolic side of the outer membrane indicating a role of cytosolic protein kinases in the regulation of mitochondrial protein import [2]. We could identify two cytosolic kinases Casein kinase 2 (CK2) and Protein kinase A (PKA) that phosphorylate Tom22 and Mim1 (CK2) and Tom70 (PKA). CK2 phosphorylates Ser44 and Ser46 of Tom22 thereby stimulating mitochondrial targeting of Tom22 as well as docking of the receptor Tom20 to the TOM-complex. Inactivation of CK2 activity causes a dramatic impairment of the fully assembled TOM-complex in vivo and consequently to reduced import rates of the major mitochondrial preprotein import pathways [3,4]. In addition we identified with CK1 a mitochondrial associated TOM kinase which mitigates PKA activity at the entry gate [3,4] and found a first link between cell cycle regulation and the mitochondrial protein import machinery component Tom6 which is phosphorylated by cyclin dependent kinases during the M-phase [5].
We are interested in the signalling processes that regulate protein import across and into the outer mitochondrial membrane and focus here on the regulation of the biogenesis pathway of beta-barrel proteins which require not only TOM but also the sorting- and assembly machinery (SAM) in the outer membrane [6].
References
[1] Schmidt O, Pfanner N, Meisinger C (2010) Nat. Rev. Mol. Cell. Biol. 11:655-667.
[2] Schmidt O, Harbauer AB, Rao S, Eyrich B, Zahedi RP, Stojanovski D, Schönfisch B, Guiard B, Sickmann A, Pfanner N, Meisinger C (2011) Cell 144:227-239.
[3] Gerbeth C, Schmidt O, Rao S, Harbauer AB, Mikropoulou D, Opalinska M, Guiard B, Pfanner N, Meisinger C (2013) Cell Metab. 18:578-587.
[4] Opalińska M and Meisinger C (2015) Curr. Opin. Cell Biol. 33: 42-48.
[5] Harbauer AB, Opalinska M, Gerbeth C, Herman JS, Rao S, Schönfisch B, Guiard B, Schmidt O, Pfanner N, Meisinger C (2014) Science 346:1109-1113.
[6] Wiedemann N, Kozjak V, Chacinska A, Schönfisch B, Rospert S, Ryan MT, Pfanner N, Meisinger C (2003) Nature 424:565-571.
Contact
Prof. Dr. Chris Meisinger
Institute of Biochemistry and Molecular Biology
Stefan-Meier-Str. 17
79104 Freiburg
Phone: +49 (761) 203 5287
Fax: +49 (761) 203 5261
christof.meisinger@biochemie.uni-freiburg.de
http://www.biochemie.uni-freiburg.de/ag-en/meisinger/meisinger/



